About Us
Driving Innovation in Drug Development
Accelerating your path to breakthrough therapies with expert guidance and tailored solutions at every stage.

PDC-CRO partners with you to drive your projects from discovery to delivery.
At PDC-CRO, we are redefining the landscape of drug development. As a forward-thinking CRO, we are more than just a service provider; we are your strategic partner in innovation. Our passionate team is comprised of industry leaders, dedicated to transforming your concepts into successful therapies. We leverage cutting-edge technology and a deep understanding of regulatory environments to ensure your projects reach their full potential.
Our commitment goes beyond typical CRO support. We are driven by a relentless pursuit of excellence and a belief in the power of collaboration. By working closely with you, we customize our approach to fit your unique needs, ensuring that every milestone is met on time and within budget. At PDC, your vision becomes our mission, and together, we push the boundaries of what is possible within clinical trials.
Vision
To become leaders in Health Data Science by applying and adhering to the highest quality of global standards and new innovations in the clinical research industry.
Mission
To utilize our experience and pool of expertise to advance the practice of clinical research in the Middle East and Africa region.
Values
To go above and beyond what is required in a project, while keeping a high professional standard of excellence, ethics and safety.
Our Approach
Guided by ingenuity, collaboration, and expertise, we tailor our approach to accelerate drug development and impactful outcomes.
Innovative Industry Experts
Our innovative team blends experience and creativity to deliver customized solutions, ensuring success at every phase.
Unique Solutions for Complex Needs
We provide one-of-a-kind solutions for complex challenges, ensuring flexible, efficient, and customized strategies for every trial.

Exceptional Client Services
We prioritize customer service, fostering strong partnerships through transparency, reliability, and personalized support for your needs.
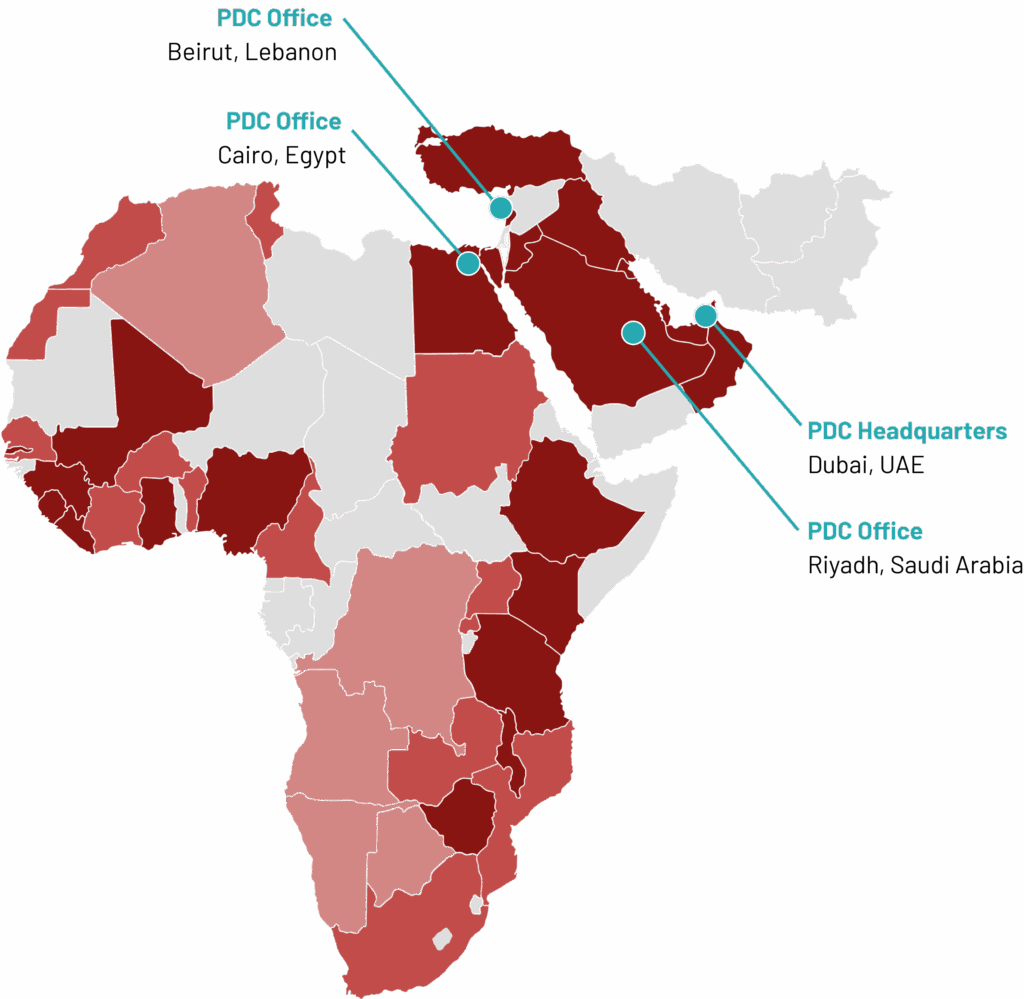
PDC Regional Coverage
PDC-CRO operates across 15+ countries in the Middle East and Africa, offering clients seamless access to diverse populations, regulatory environments, and clinical infrastructure. Our deep regional knowledge, combined with global research standards, allows us to navigate local complexities while ensuring high-quality trial execution. With a strong on-the-ground presence and trusted site relationships, we are uniquely positioned to deliver results across the MEA region.
- PDC Office Location
- Countries covered through PDC Resources
- Countries covered through PDC-cross border resources
- Countries covered through subcontractor CRO’s
Leadership Team
Mohamed Mostafa is the Chief Executive Officer of PDC-CRO and serves on its Board of Directors. Mohamed leads a workforce that supports pharmaceuticals, biotech companies, and research institutions in clinical development and transformational research. Mohamed’s inspiration and passion for clinical research led him to focus on how clinical research can improve patients’ lives. Over two decades of versed experience, he dedicated his efforts to expand research capabilities and infrastructure to provide patients an option to access new treatments.
PDC is a leading CRO in the Middle East and Africa Region that focuses on early-stage clinical development, operational management of clinical trials, and regulatory services. More recently, the company’s rapid response to the COVID-19 pandemic drove a coordination of global and regional initiatives to safeguard the health of people and ensure vaccines, life-saving medicines, and products reach patients and communities in need.
Mohamed holds a bachelor’s degree in Pharmaceutical Science from Egypt and is currently responsible for the overall management and strategic initiatives within the organization. Prior to that, he was heading the Clinical Operations across different CROs and Pharmaceutical Companies like Novartis and Roche. In his capacity, he was responsible for various scientific initiatives with local and international partners and worked closely with regulatory agencies across the region on various guidelines and strategic plans.

Rania AlShami
Chief Business and Strategy Officer
Dr. Rania Al Shami is Chief Business and Strategy Officer at PDC CRO; as such, she is responsible for Business Development and strategic initiatives within the organization. Rania holds a Doctorate degree in Cell Biology and an MBA from the University of Delaware in the USA. Dr. Al Shami has more than 15 years of experience in the CRO industry in the MENA region.
She joined from CTI MEA (Clinart), where she acted as VP of Business Development for 7 years. Prior to that, Dr. Al Shami acted as Managing Director for MENA at Ergomed Clinical Research and was responsible for setting up and managing the company’s Middle East and North Africa (MENA) region activities from the regional headquarters office based in Dubai, UAE. Before joining Ergomed as Director of Business Development, Rania had worked as Associate Director for Science Projects at DuBiotech (Dubai Science Park), a Dubai government initiative dedicated to establishing a Life Science industry in the region. She was responsible for various scientific investment initiatives with local and international partners and worked closely with regulatory agencies across the region on various guidelines and strategic plans.
Rania has a solid understanding of the MENA region’s Pharma, Biotech, and Healthcare markets and continues to work closely with colleagues to further develop the clinical research capabilities within the MENA region.

Monika Atanassova
Chief Quality Officer
Monika Atanassova brings over 23 years of extensive international experience in clinical research, encompassing roles in Quality Assurance, Audit, Corporate Strategy and Planning, Executive Leadership, and Human Resources.
She has been instrumental in the exponential growth of one of Bulgaria’s pioneering and leading full-service CRO. Through her significant contributions and key leadership roles, Monika has overseen the company’s expansion from 8 to over 280 employees and the establishment of offices in 8 international locations, covering 14 countries in Eastern Europe.
Since 2015, she has been engaged in international consultancy in Quality Management, Auditing, and Corporate Strategy and Planning across Europe and the emerging MENA market. For four years, Monika Atanassova was part of the Bulgarian team of the largest Site Network Organization, focusing on compliance and inspection readiness.
In 2020, she joined PDC, contributing to its rapid expansion and success. Under her guidance, the organization has continued to thrive, implementing initiatives that have significantly enhanced its quality, robustness, and compliance reach.
Monika Atanassova holds a master’s degree in pharmacy with distinction from the Medical University, Sofia, and a master’s degree in clinical trial management. She has been a member of the Research Quality Association (RQA) since 2008.

Fady Saad
Chief Operating Officer - Clinical Research
Fady joined PDC as Chief Operating Officer (COO) in January 2024, bringing a wealth of expertise in the pharmaceutical industry and a strong focus on operational excellence. As a key member of PDC’s executive management team, he plays a pivotal role in shaping the company’s mission, vision, and strategic goals, while setting high standards for quality, business conduct, and ethics across the organization.
Fady oversees the execution of PDC’s operations, ensuring seamless communication and collaboration with sponsors, partner CROs, physicians, and regulatory authorities. He is responsible for ensuring all activities are executed in accordance with predefined plans, meeting contractual obligations and regulatory requirements. Additionally, Fady provides leadership in mentoring, training, and tracking the performance of key divisions, including Regulatory & Start-Up, Project Management, and Clinical Operations, while working closely with Finance, Quality, and Business Development departments.
Fady holds a Doctor of Pharmacy degree from the Lebanese University and a Master’s Degree in Public Health. With deep expertise in the pharmaceutical sector, he has a strong focus on Research & Development, as well as building robust medical and scientific capabilities within organizations.
Prior to joining PDC, Fady led Novartis’ R&D portfolio across the Middle East and North Africa (MENA) region for over 10 years, where he successfully expanded operations into new countries and institutions, significantly increasing the region’s contribution to various phases of clinical trials. His experience spans multiple therapeutic areas, including oncology, hematology, rare diseases, neuroscience, cardiology, and endocrinology.
Throughout his career, Fady has built and led high-performing clinical research teams in the MENA region, focusing on research and medical training, budget and strategic planning, operational excellence, and portfolio and business development. He is recognized as a dedicated team player and a skilled communicator, known for his ability to balance both internal and external priorities and eliminate barriers across diverse, multi-cultural environments.
Fady is also a passionate customer advocate, consistently working to deliver value while ensuring that organizational and client goals are met with efficiency and precision.

Rita Ojeil
Chief Operating Officer - HEOR & Consultancy
With over 20 years of leadership in the MENA pharmaceutical and consulting industries, Rita Ojeil is a distinguished expert in Health Economics and Outcomes Research (HEOR), Market Access, and Health Policy. As the Chief Operating Officer of Carexso FZ LLC, she drives strategic initiatives across the Middle East and Africa (MEA), leading large-scale projects that shape healthcare access, pricing strategies, and real-world evidence generation.
Rita’s expertise spans economic modeling, HTA submissions, value communication, and Real-world evidence. She has directed more than 200 HEOR evaluations, including Budget Impact Analyses (BIAs), Cost-Effectiveness Analyses (CEAs), and cost-of-illness studies. Her leadership has also facilitated the deployment of over 30 patient support programs and 45 managed entry agreements, particularly in Oncology, Hematology, Neurology, Rare Diseases, and Immunology.
A thought leader in healthcare transformation, Rita has played a pivotal role in Saudi Arabia’s EQ-5D-5L valuation study and Multi-Criteria Decision Analysis (MCDA) initiatives, contributing to 18+ peer-reviewed publications, including manuscripts, white papers, and conference posters. Her deep understanding of healthcare systems and reimbursement frameworks makes her a sought-after voice in market access and health policy discussions.
Fluent in English, French, Arabic, and Spanish, Rita excels in cross-border collaborations, bridging gaps between stakeholders to drive innovative, value-driven healthcare solutions.