From Innovation
To Impact
Experts in early-to-late phase drug development services—offering customized, end-to-end solutions
Innovating Drug Development
At PDC-CRO, we go beyond being a full-service CRO—we are dedicated partners in clinical research. With expertise spanning Phase I to Phase IV trials, our team works closely with clients to navigate the complexities of drug development with agility and precision. Whether as part of an integrated program or a stand-alone solution, we tailor our approach to meet your needs, ensuring flexibility, efficiency, and the highest standards of quality to keep your milestones on track and on budget.
Clinical Operations
Efficient trial execution to manage trial planning, logistics, and reporting—ensuring timely, cost-effective studies
Regulatory Services
Expert regulatory guidance to navigate complex regulations, ensuring compliance and timely submissions.
Site Management & Coordination
Streamlined site operations to efficiently manage site selection, patient recruitment, and monitoring for seamless clinical trials.
Clinical Outsourcing & Strategic Partnership
Flexible support to tailor clinical outsourcing solutions that scale to your project’s specific needs.
Data Management
Advanced data capture systems that transforms complex datasets into actionable insights for informed decisions.
Biostatistical Support
Robust support to provide reliable statistical analysis and study design for sound, submission-ready results.
Medical Writing
Clear, compliant documentation to create accurate, regulatory-compliant documents from protocols to clinical study reports.
Clinical Monitoring
Proactive, risk-based oversight that ensures compliance, patient safety, and timely, high-quality clinical trial outcomes.
Capacity Building
Targeted training, SOP development, and regulatory collaboration to enhance local expertise and strengthen your clinical research infrastructure.
Pharmacovigilance
Comprehensive, end-to-end safety monitoring solutions that ensure patient welfare, regulatory compliance, and effective risk management.
Consulting & Analytics
Strategic consulting and analytics solutions that leverage real-world data, health economics, and regulatory expertise to drive informed, policy-aligned commercial decisions.
Health Economics & Outcomes Research & Health Policy
Value-driven insights demonstrate cost-effectiveness and long-term benefits with our expert health economics analysis.
Real-World Evidence & Site Management
Integrated real-world evidence and site management solutions that deliver high-quality data to support regulatory approval, market access, and clinical outcomes.
Therapeutic Areas
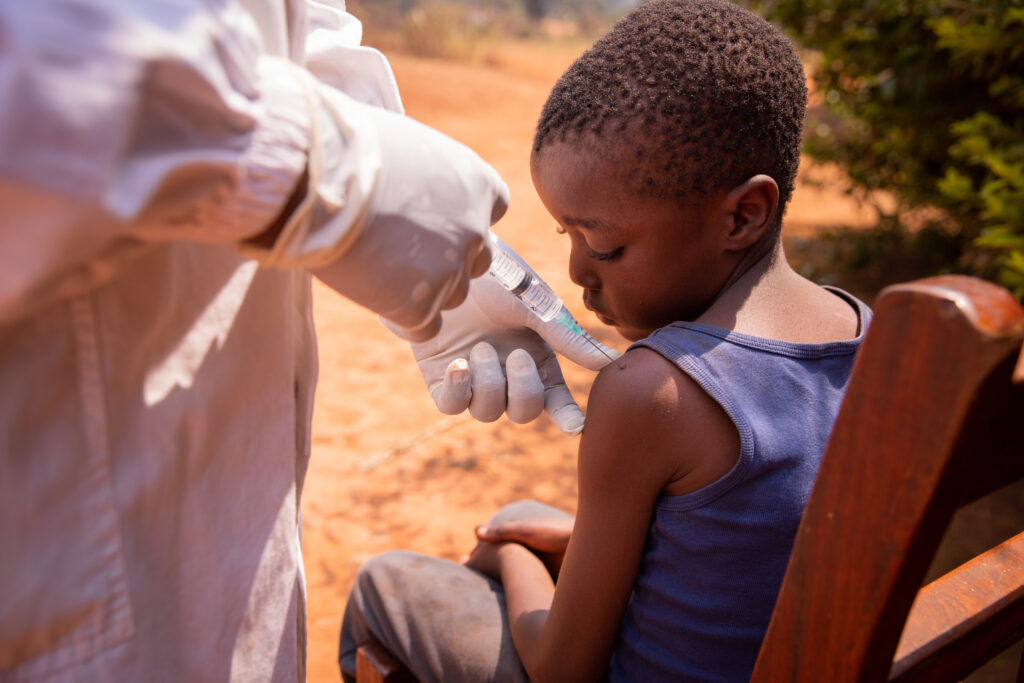
Infectious Disease
PDC-CRO has extensive experience conducting vaccine and treatment trials for infectious diseases, including COVID-19, Meningitis, HPV, MERS, and Hepatitis C, across multiple regions.
Rare Disease
Our team specializes in rare disease clinical trials, leveraging deep regulatory expertise and patient-centered strategies to advance research in underserved therapeutic areas.
Hematology/Oncology
With a robust track record in hematology and oncology trials, PDC-CRO has conducted numerous studies across phases I-IV, supporting innovative cancer treatments in the MEA region.
Metabolic Disorders
We provide tailored clinical research solutions for metabolic disorders, ensuring comprehensive study design and execution to advance novel therapies for conditions such as diabetes and lipid disorders.
Cardiovascular Disease
PDC-CRO supports cardiovascular clinical trials with a focus on precision, regulatory compliance, and patient safety, addressing a range of heart and vascular conditions.